ST. LOUIS — In the race to get the country vaccinated, clearing the hurdles that come with FDA approval is a big boost for Pfizer's COVID-19 shot. But it's not all cheers from the crowd, with some now questioning how quickly the process went.
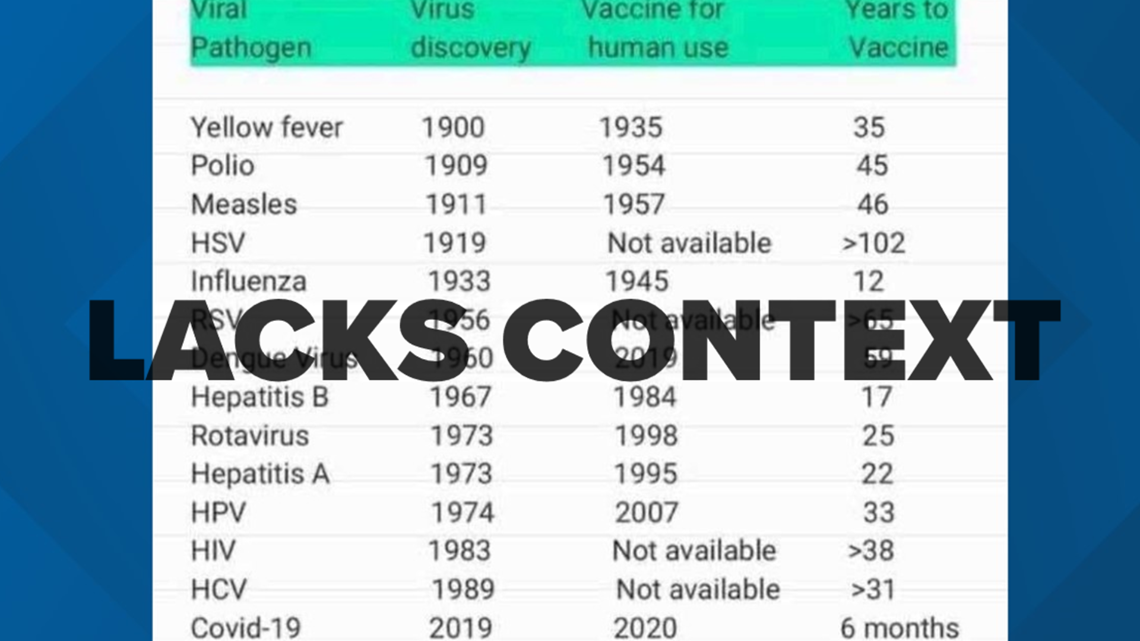
A graphic circulating online juxtaposes the timelines from when a virus was detected to when it a vaccine was developed, intending to highlight the short timeline for the development of the COVID-19 vaccines.
However, Washington University vaccine expert Dr. Michael Kinch said the timelines aren’t painting an accurate picture of how shot development works.
“The logic is backwards, frankly, what those long time periods reflect is our first of all inability to identify and link the particular pathogen particular organism to the disease,” he said, meaning sometimes it takes years to understand what's causing a particular illness before scientists can even begin developing a shot.
With COVID-19, researchers quickly correctly identified it as a coronavirus -something that can often cause a common cold - and paired it with the vaccine and MRNA technology they'd been exploring for years.
“In the case of this virus, we have the right technology partnered with the right target and they came together beautifully,” said Dr. Kinch.
COVID-19 is also unique because it spreads people at such a rapid pace, which actually helped speed up vaccine testing. Scientists could gather more than enough data to show them the vaccine is safe and effective, over more than enough observation time than needed, before Pfizer asked the FDA for full approval.
While this is the FDA’s fastest vaccine approval ever, even this part wasn't exactly "rushed," more like "fast-tracked," Dr. Kinch said.
"It's known as an expedited review, but in this case, they sort of super expedited it," said Dr. Kinch. "They put all hands on deck everybody has to review this."
The approval process means slowly wading through piles of paperwork, something you don't have a lot of time for during a global pandemic. Instead of skipping steps, the FDA essentially put a lot more people and resources into the process right away.
“It's shockingly safe and it's amazingly efficacious,” said Dr. Kinch of their findings. “They had a lot to review, but they took the job very, very seriously and I have no concerns that any corners were cut.”
The Pfizer covid-19 vaccine didn't breeze through the safety requirements to meet FDA approval because getting out of this pandemic is still a marathon and a science.
This unsourced graph is circulating online as people question the timeline for Pfizer's COVID-19 vaccine approval. However, Dr. Kinch said the logic is backward and misleading.