ST. LOUIS — St. Louis health officials said respiratory syncytial virus, or RSV cases, are filling up children's hospitals in the area.
Fortunately, there could be a potential vaccine to prevent the virus.
On Tuesday, Pfizer announced promising data about its recent trials for the first maternal RSV vaccine. One of those clinical studies happened right here in St. Louis.
Dr. Carol Kao, a Washington University Infectious Disease Physician at St. Louis Children's Hospital, oversaw the study at Washington University.
"It will be a lifeline for pediatricians who deal with RSV each winter," She said. "It's going to be a game changer for the RSV field because currently there are no vaccines or effective therapeutics for treatment and prevention for RSV."
A small group of pregnant St. Louis moms in the second or third trimester enrolled. They were part of just 7,400 participants worldwide.
"Phase three, randomized, double-blinded, placebo-controlled vaccine trial started in June of 2020. We followed the moms for six months and followed the babies for at least one year," Dr. Kao says.
Results from Pfizer show:
- The vaccine, when given to pregnant women, protected their babies from getting severe RSV up to 80% in the first three months of life and Dr. Kao said up to 70% in the first six months
- It also cut a baby’s risk of needing to see a doctor for an RSV infection in half
Pfizer said maternal immunization is an important way to help protect vulnerable infants when they are too young to make their own antibodies.
Starting immunization in the second trimester of pregnancy, protective antibodies are naturally passed from the mother’s circulation across the placenta and to the developing fetus.
It comes at a time when RSV cases are spiking.
The St. Louis Pandemic Task Force hosted a briefing about the issue Tuesday.
"Children’s hospitals are getting full and that combined with an expected heavy flu season and COVID, it's the tripledemic people talk about," said Dr. Marya Strand, Chief Medical Officer at SSM Health Cardinal Glennon Children’s Hospital.
Dr. Strand said more children are needing a higher level of treatment. Plus, emergency rooms have been exceedingly busy and will be for the next few weeks.
The health officials are urging parents to look at their children's symptoms and see what the next best steps are.

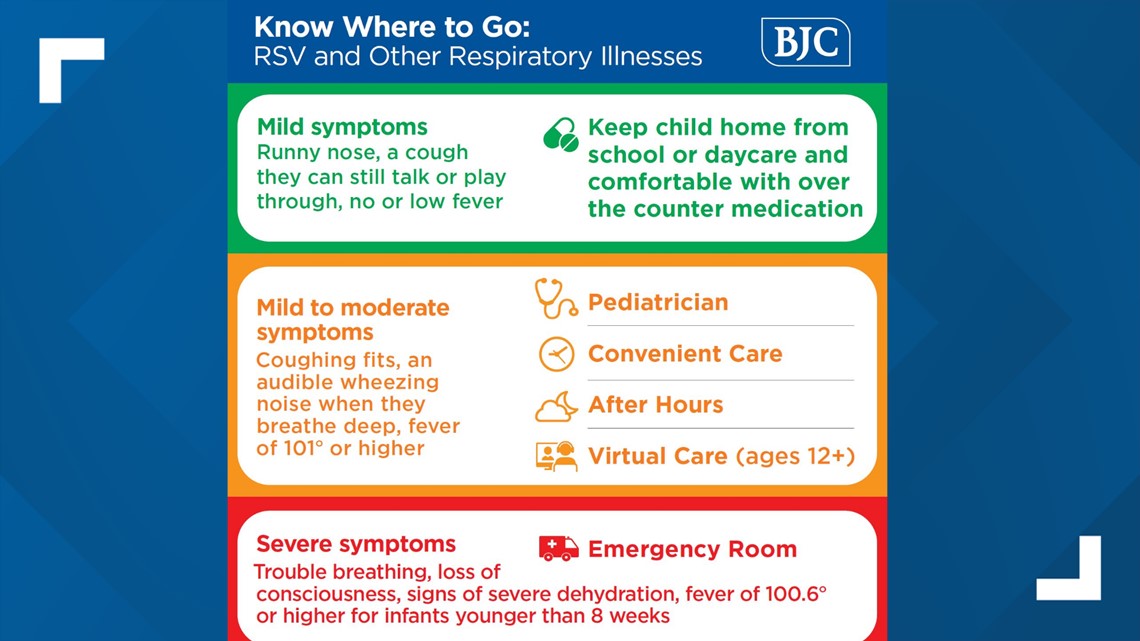
Dr. Kao said she worries about the littlest ones.
"In young babies, it can cause severe disease because of their small airways. It's the number one cause of hospitalizations for infants in the U.S.," Dr. Kao said.
She said she believes if the vaccine is approved now, it could impact the future.
"If it could be available for next season, I think we could see a very different RSV season next year," Dr. Kao said.
Pfizer plans to submit for FDA approval by the end of the year.
If approved, Pfizer's vaccine will be the first to prevent RSV infection.

