ST. LOUIS — You might be wondering if that cough or ache you had in February was COVID-19, before widespread testing was available. It’s possible, and it’s worth finding out.
Knowing whether you had COVID-19 and recovered could mean immunity for the future. Plus, you could donate your blood to help other people recover from the virus. But if you didn’t get a test while experiencing symptoms, is it possible to know whether you were exposed to SARS-CoV-2?
An infectious disease specialist from SSM Health has a word of caution about the set of tests being offered to the public that claim to look for COVID-19 antibodies.
Dr. Alex Lacasse said the tests on the market now have not been approved by the FDA.
“We don't have a very, very solid test right now,” said Lacasse. “I think there's been a lot of money involved in this and a lot of companies that want to be part of the market.”
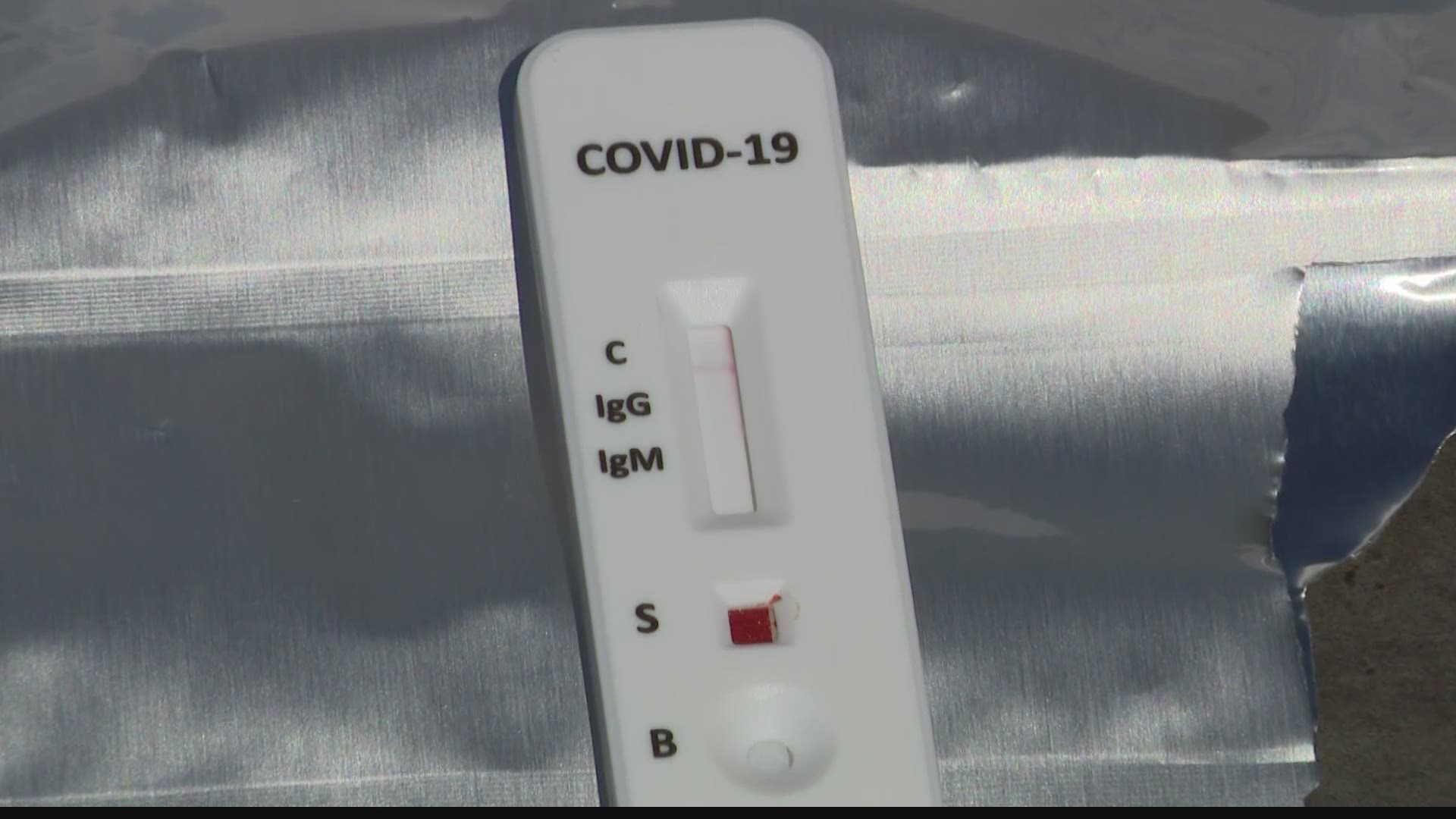
Two kinds of tests with very different results
Antibody tests are different from what doctors use to determine if you’re sick from COVID-19 right now. The tests given at drive-thru testing sites and hospitals are usually live virus tests. The health care provider sticks a swab way up into the patient’s sinuses to find out if there’s literally the COVID-19 virus living there. If the swab has copies of the genetic material (RNA) from the virus, the patient knows that they were exposed.
Antibody tests are supposed to find what’s left over after the body gets rid of the SARS-CoV-2 virus. They look for evidence in the blood that the patient’s body produced antibodies that are specifically designed to kill SARS-CoV-2. Having those antibodies usually means immunity from any more infections for that specific strain, but there hasn’t been much research on whether those antibodies stick around and how effective they are.
“We know they can neutralize the infection. The question is for how long and we don't know the answer to that. I mean, time will tell us if it's going to be a lifelong immunity or not,” said Lacasse.
Antibodies for some viruses, like smallpox, can stick around for decades or more. But some diseases require regular re-exposure, which is why doctors recommend that patients get a tetanus shot every decade.
What can an antibody test tell you?
Lacasse said about 150 companies are offering an antibody test that claims to detect coronavirus antibodies. Some of these specifically claim to detect the antibodies for COVID-19. Yet many antibody tests offered at commercial labs are not testing for a specific strain. Instead, they’re looking for antibodies for related strains of coronavirus.
Arcpoint Labs in Creve Coeur is offering an antibody test for around $200. The lab’s president, Scott Lambert, wrote that tests they offer “are not able to distinguish whether the antibodies were generated in response to COVID-19 or several other non-SARS-COV-2 coronavirus strains such as HKU1, NL63, OC43 or 229E.”
He previously told the I-Team’s PJ Randhawa that the presence of antibodies from another strain of coronavirus may provide a level of protection from COVID-19.
Lacasse said there isn’t science to back up that claim yet.
“It's saying 'we don't have a great test,’” said Lacasse. “Coronavirus has been here since at least the ‘70s when it was first described. So, saying there's partial immunity from the other viruses that were in circulation for 50 years — why do we have an outbreak right now with COVID-19? If we had partial immunity or a partial response to it, why are we seeing so many people infected and having severe disease?”
That means that a positive antibody test doesn’t say enough about immunity, and making decisions based on those results could be dangerous.
“That's the difficulty about the tests, and tests that have to be developed very quickly. We don't want to develop that so quickly that we won't have a test that's going to be really helpful to both the clinician and especially the patient,” said Lacasse.
Antibody tests find use in hospitals
As of April 29, the FDA has authorized the use of about 50 tests for COVID-19 on an emergency basis. Only eight of them are antibody tests using blood. An emergency-use authorization is different from FDA approval because the authorization ends when the FDA decides the emergency is over.
That’s why Dr. Lacasse recommended that the best use of antibody tests now is in trials for additional research.
“I’m an infectious disease doctor and I'm telling you not to get a test about an infectious disease process,” said Lacasse.
SSM Health is evaluating an antibody test now, but not for diagnostic purposes. An SSM Health representative wrote that they will be rolling out a test with emergency authorization from the FDA to the hospital system as they study how many people have been infected that were never tested. They added that doctors don’t yet know if having a COVID-19 antibody means someone is immune.